bort medical 054 300 Instructions For Use Manual
- Typ
- Instructions For Use Manual

BORT. Das Plus an Ihrer Seite.
Gebrauchsanweisung
BORT Fußheberorthese
054 300

Illustrationen
Figures
C
B
A
C
B
A
C
B
A
C

DE
deutsch Gebrauchsanweisung 04
E
english Instructions for use 09
FR
français Mode d’emploi 14
ES
español Instrucciones de uso 19
IT
italiano Instruzioni per l’uso 24
L
nederlands Gebruiksaanwijzing 29
CS
český Návod k použití 34
ET
eesti Kasutusjuhend 38
PL
polski Instrukcja użytkowania 42
RO
românesc Instrucțiunile de utilizare 47
PDF: ga.bort.com
Sprachen
Languages

04
DE
Vielen Dank für das Vertrauen in ein Medizinprodukt der BORT GmbH. Bitte lesen Sie
die vorliegende Gebrauchsanweisung sorgfältig durch. Bei Fragen wenden Sie sich
an Ihren Arzt oder Fachhandel von dem Sie dieses Medizinprodukt erhalten haben.
Zweckbestimmung
Bei diesem Medizinprodukt handelt es sich um eine Fußheberorthese aus
elastischem Material und festen textilen Bestandteilen. Stabilisierungselemente und
Korrekturzügeln unterstützen die Fußhebermuskulatur bei inkompletter Lähmung.
Indikationen
Peroneusparese, bei allen Ausprägungsgraden, besonders für leichte bis mittlere,
Fuß- und Zehenheberparesen (Kraftgrad 2 – 4).
Kontraindikationen
Thrombosegefahr, hochgradige Varikosis, periphere arterielle Verschlusskrankheit
(PAVK), Lymphabflussstörungen auch unklare Weichteilschwellungen körperfern des
angelegten Hilfsmittels, Empfindungs- und Durchblutungsstörungen der versorgten
Körperregion, Erkrankungen der Haut im versorgten Körperabschnitt, Latexallergie.
Anwendungsrisiken / Wichtige Hinweise
Dieses Medizinprodukt ist ein verordnungsfähiges Produkt. Sprechen Sie
Anwendung und Dauer mit Ihrem verordnenden Arzt ab. Die Auswahl der geeigneten
Größe und eine Einweisung erfolgt durch das Fachpersonal, von dem Sie das
Medizinprodukt erhalten haben.
– Medizinprodukt vor radiologischen Untersuchungen ablegen
– Wurde das Tragen bei Nacht angeordnet, Beeinträchtigungen des Blutkreislaufs
vermeiden.
– bei Taubheitsgefühl Medizinprodukt lockern oder ggf. abnehmen
– bei anhaltenden Beschwerden den Arzt oder Fachhandel aufsuchen
– Medizinprodukt indikationsgerecht einsetzen
– gleichzeitige Nutzung anderer Produkte nur nach Rücksprache mit Ihrem Arzt
– keine Änderungen am Produkt vornehmen
– nicht auf oenen Wunden tragen
BORT Fußheberorthese
DE

05
DE
– nicht verwenden bei Unverträglichkeiten gegen eines der verwendeten Materialien
– kein Wiedereinsatz – dieses Hilfsmittel ist zur Versorgung eines Patienten
bestimmt
– während der Tragedauer der Orthese: keine lokale Anwendung von Cremes oder
Salben im Bereich des angelegten Hilfsmittels – kann Material zerstören.
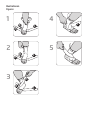
Anziehanleitung
Vorbereitung zum Anlegen der BORT Fußheberorthese:
Die Fußheberorthese kann direkt auf der Haut sowie über einem dünnen Strumpf
getragen werden. Idealerweise legen Sie die Fußheberorthese im Sitzen an.
1. Önen Sie alle Klettverschlüsse der Fußheberorthese und ziehen Sie die
Fußheberorthese über Ihren Fuß.
2. Greifen Sie in die Fingerschlaufe, die an der Rückseite der Fußheberorthese am
oberen Abschlussrand befestigt ist und ziehen Sie die Fußheberorthese über die
Ferse nach oben.
Schließen der Klettverschlüsse und Anlegen der Zügel:
Bitte achten Sie vor dem Schließen der Klettzügel darauf, dass die Fußheberorthese
richtig am Fuß positioniert ist. Die Fußheberorthese muss an der Ferse korrekt
anliegen. Für einen wirkungsvollen Zügelverlauf müssen die Nähte am Fußrücken
und im Fersenbereich mittig verlaufen.
Wichtig: Der Fuß bleibt über den gesamten nachfolgenden Anlegevorgang der
Fußheberorthese mit der Ferse auf dem Boden!
Stellen Sie den Fuß auf den Boden. Legen Sie die Zügel A und B auf der
Außenseite, Zügel C auf der Innenseite des Fußes auf den Boden.
Schließen Sie nun die Klettverschlüsse am Unterschenkel in folgender
Reihenfolge:
– Den unteren der drei Verschlüsse zuerst.
– Im nächsten Schritte den Verschluss am oberen Abschlussrand.
– Abschließend den mittig positionierten Verschluss.

06
DE
Passen Sie bei Bedarf den Zug der Verschlüsse am Unterschenkelteil der
Fußheberorthese nochmals an, um einen angenehmen, aber stabilen Halt der
Fußheberorthese am Unterschenkel zu erreichen.
Schieben Sie den Oberschenkel bzw. das Knie so weit wie möglich nach
vorne. Ideal ist ein Winkel des Fußes von 20 – 30° zum Unterschenkel hin.
Führen Sie den Zügel A zuerst senkrecht nach oben, dann unter Zug schräg über
den Fußrücken und oberhalb des Innenknöchels um den Unterschenkel. Kletten Sie
den Zügel auf dem rückseitig angebrachten Hakenband fest.
Führen Sie den unelastischen Zügel B an der Außenseite des Fußes unter
Zug nach oben. Heben Sie dabei den Fußaußenrand mit dem Zügel etwas an.
Befestigen Sie dann Zügel B auf der Velours-Klettfläche der Fußheberorthese.
Wichtig: Zügel B muss senkrecht nach oben verlaufen. Er stabilisiert das
Sprunggelenk und soll ein Umknicken nach außen beim Gehen verhindern.
Abschließend schließen Sie den Zügel C. Führen Sie Zügel C zuerst senkrecht
nach oben, unter Zug schräg über den Fußrücken und oberhalb des
Außenknöchels um den Unterschenkel. Dann befestigen Sie den Zügel C auf dem
rückseitig angebrachten Hakenband auf Zügel A. Zügel C möglichst deckungsgleich
auf dem Hakenband von Zügel A festkletten um Beschädigungen an Strümpfen zu
vermeiden.
Ablegen
Zum Ablegen der BORT Fußheberorthese önen Sie die Gurte in umgekehrter
Reihenfolge.
Tipp: Schließen Sie die Klettverschlüsse um einen frühzeitigen Verschleiß der
Klettfläche zu vermeiden.

07
DE
Materialzusammensetzung
Polyamid (PA), Elastodien (ED), Viskose (CV)
Die genaue Materialzusammensetzung entnehmen Sie bitte dem eingenähten
Textiletikett.
Das Produkt enthält Latex und kann allergische Reaktionen auslösen.
Reinigungshinweise
Schonwaschgang Nicht chemisch reinigen Nicht bleichen
Nicht im Wäschetrockner trocknen Nicht bügeln
Keinen Weichspüler verwenden. In Form ziehen und an der Luft trocknen.
Klettverschlüsse schließen, um die Beschädigung anderer Wäschestücke zu vermeiden.
Gewährleistung
Für das erworbene Produkt gelten die gesetzlichen Bestimmungen des Landes, in
dem Sie das Produkt bezogen haben. Bitte wenden Sie sich an Ihren Fachhandel,
sollten Sie einen Gewährleistungsfall vermuten. Bitte das Produkt vor Einreichung
eines Gewährleistungsfalles reinigen. Wurden beiliegende Hinweise der
Gebrauchsanweisung nicht ausreichend beachtet, so kann die Gewährleistung
beeinträchtigt werden bzw. entfallen. Ausgeschlossen ist eine Gewährleistung bei
nicht indikationsgerechter Anwendung, Nichtbeachtung der Anwendungsrisiken,
-hinweise sowie eigenmächtig vorgenommenen Änderungen am Produkt.
Nutzungsdauer / Lebensdauer des Produkts
Die Lebensdauer des Medizinprodukts wird durch den natürlichen Verschleiß bei
sach- und anwendungsgemäßem Umgang bestimmt.

08
DE
Meldepflicht
Kommt es bei der Anwendung des Medizinproduktes zu einer schwerwiegenden
Verschlechterung des Gesundheitszustandes, dann melden Sie dies Ihrem
Fachhändler oder uns als Hersteller sowie dem BfArM (Bundesinstitut für
Arzneimittel und Medizinprodukte).
Unsere Kontaktdaten entnehmen Sie der vorliegenden Gebrauchsanweisung. Die
Kontaktdaten der benannten Stelle Ihres Landes finden Sie unter folgendem Link:
www.bort.com/md-eu-kontakt.
Entsorgung
Nach Nutzungsende muss das Produkt entsprechend örtlicher Vorgaben entsorgt
werden.
Konformitätserklärung
Wir bestätigen, dass dieses Produkt den Anforderungen der VERORDNUNG (EU)
2017/745 DES EUROPÄISCHEN PARLAMENTS UND DES RATES entspricht. Die
aktuelle Konformitätserklärung finden Sie unter folgendem Link:
www.bort.com/konformitaet
Stand: 10.2019
Medizinprodukt |
Einzelner Patient – mehrfach anwendbar

09
E
Many thanks for placing your trust in a medical device from BORT GmbH. Please
read the existing instructions for use carefully. If you have any questions, please
refer to your physician or the specialist retailer from whom you purchased this
medical device.
Intended purpose
This medical device is a tibialis anterior muscle brace consisting of elastic material
and rigid textile components. Stabilisation elements and correction straps support
the tibialis anterior muscle in case of partial paralysis.
Indications
Peroneal paralysis of any severity, especially for mild to medium paralysis of the
tibialis anterior muscle and the flexor hallucis longus (degree of severity 2 – 4).
Contraindications
Thrombosis risk, extreme varicosis, peripheral arterial occlusive disease (PAOD),
lymph drainage disorders, also unclear soft tissue swellings distal to the aid
positioned, sensory and circulatory disorders of the region of the body treated, skin
diseases in the part of the body treated, latex allergy.
Application risks/Important notes
This medical device is a prescribable product. Discuss the use and duration with
your treating physician. The expert sta from whom you have received the medical
device will select the appropriate size and instruct you regarding its use.
– remove the medical device prior to radiological examinations
– if wearing at night is prescribed, avoid negative influence on the circulatory system
– in case of numbness, loosen the medical device and remove it if necessary
– in case of persistent complaints, consult the physician or specialist retailer
– use the medical device in accordance with therapeutic needs
– only use other products simultaneously after consultation with your physician
– do not make any changes to the product
– do not wear it on open wounds
– do not use in case of intolerance of one of the materials used
BORT Foot Levator Orthotic Device
E

10
E
– no re-use – this medical aid is intended for treating one patient
– whilst wearing the brace, please neither use any creams nor ointments on our
around it as they can destroy the material
Fitting instructions
Preparation for putting the BORT tibialis anterior muscle brace on:
The tibialis anterior muscle brace can be worn directly on the skin and over a thin
sock. It’s best to put the brace on when sitting.
1. Open all the tibialis anterior muscle brace Velcro fasteners and pull the brace
over your foot.
2. Grasp the finger loop attached to the reverse side of the tibialis anterior muscle
brace on the upper material edge and pull the tibialis anterior muscle brace
upwards over the heel.
Closing the Velcro fasteners and attaching the restraints:
Before closing the Velcro constraints, please observe that the tibialis anterior muscle
brace is correctly positioned on the foot. The tibialis anterior muscle brace must be
correctly placed on the heel. For an eective course of the restraint, the seams must
be central on the instep and in the heel area.
Important: During the entire following attachment process for the tibialis anterior
muscle brace, the foot and heel remain on the floor!
Place your foot on the floor. Place restraints A and B on the outer side and
restraint C on the inner side of the foot on the floor.
Then close the Velcro fasteners on the lower leg in the following
order:
– Firstly, the lower of the three fasteners.
– In the next step, the fastener on the upper material edge.
– Finally the fastener positioned in the middle.

11
E
If necessary, adjust the tension of the fasteners on the lower leg part of the tibialis
anterior muscle brace again in order to achieve a pleasant but stable hold of the
tibialis anterior muscle brace on the lower leg.
Slide the thigh or the knee as far forwards as possible. A foot angle of
20 – 30° to the lower leg is ideal. Guide restraint A upwards vertically first,
then under tension diagonally over the instep and above the inner ankle around the
lower leg. Velcro the restraint to the hook strap attached on the reverse side.
Guide the non-elastic restraint B upwards on the outer side of the foot under
tension. At the same time, raise the outer edge of the foot slightly with the
restraint. Then, attach restraint B to the velour Velcro surface of the tibialis anterior
muscle brace.
Important: Restraint B must run upwards vertically. It stabilises the ankle joint and
is intended to hinder twisting outwards when walking.
Finally, close restraint C. First, guide restraint C vertically upwards under
tension diagonally over the instep and above the lateral malleolus around
the lower leg. Then, attach restraint C to the hook ribbon attached to the rear side
on restraint A. Attach restraint C to the restraint A hook strap as congruently as
possible in order to avoid damages to socks.
Removal
To remove the BORT tibialis anterior muscle brace, open the belts in the reverse order.
Tip: Close the Velcro fasteners in order to avoid early wear to the Velcro surface.
Material composition
Polyamide (PA), elastodiene (ED), viscose (CV)
The sewn in textile label provides the precise material composition.
The product contains latex and can trigger allergic reactions.

12
E
Cleaning information
Delicate wash Do not clean chemically Do not bleach
Do not dry in a tumble dryer Do not iron
Do not use fabric conditioner. Stretch back into shape and dry in the fresh air. Close
the Velcro fastenings to avoid damage to other laundry items.
Guarantee
The legal regulations of the country in which you acquired the product apply to the
purchased product. Please contact your specialist retailer if you suspect a warranty
claim. Please clean the product before submitting a warranty claim. If the enclosed
instructions for use have not been properly observed, the warranty may be impaired
or cancelled. The warranty does not cover use of the product inappropriate for
the indication, non-observance of application risks, instructions and unauthorised
modifications to the product.
Useful life/Lifetime of the product
The lifetime of the medical device is determined by its natural wear and tear if
treated appropriately and as recommended.
Duty of notification
If a serious worsening of the state of health occurs when using the medical device,
you are obliged to notify the specialist dealer or us as the manufacturer and the
Medicines & Healthcare products Regulatory Agency (MHRA).
You can find our contact information in these instructions for use. You can find the
contact information for the appointed authority for your country under the following
link: www.bort.com/md-eu-contact.
Disposal
Upon the termination of use, the product must be disposed of in accordance with
the corresponding local requirements.

13
E
Declaration of conformity
We confirm that this device conforms with the requirements of REGULATION (EU)
2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL. You can find the
current declaration of conformity under the following link: www.bort.com/conformity
Status: 10.2019
Medical device |
Single patient – multiple use

14
FR
Nous vous remercions de la confiance que vous accordez à l’un des dispositifs
médicaux de la société BORT GmbH. Veuillez lire attentivement l’intégralité du
présent mode d’emploi. En cas de question, consultez votre médecin ou le magasin
spécialisé qui vous a fourni ce dispositif médical.
Utilisation prévue
Ce dispositif médical est une orthèse releveur de pied composée d’une matériau
élastique et d’éléments textiles fixes. En cas de paralysie non complète, les
éléments de stabilisation et les sangles correctives aident la musculature permettant
de relever le pied.
Indications
Parésie du péroné, de tout degré de gravité, notamment pour les parésies légères à
moyenne du releveur du pied et des orteilss (force 2 – 4).
Contre-indications
Risque de thrombose, varices importantes, artériopathie oblitérante périphérique
(AOP), troubles de la circulation lymphatique y compris tuméfaction des tissus
mous d’origine inconnue éloignée de l’aide posée, troubles de sensation et de la
circulation sanguine des régions du corps traitées, maladies cutanées sur les zones
traitées, allergie au latex.
Risques inhérents à l’utilisation/Remarques importantes
Ce dispositif médical est un produit prescrit sur ordonnance. Consultez votre
médecin prescripteur pour en connaître l’utilisation et la durée de port. Le choix
de la taille ainsi qu’une présentation du produit seront réalisés par le personnel
spécialisé qui vous fournira ce dispositif médical.
– retirer le dispositif médical avant les examens radiologiques
– en cas de prescription de port de nuit, éviter toute entrave à la circulation sanguine
– en cas de sensation d’engourdissement, desserrer ou retirer éventuellement le
dispositif médical
– consulter un médecin ou un magasin spécialisé en cas de gêne persistante
– utiliser le dispositif médical conformément aux indications
BORT bandage de releveur de pied
FR

15
FR
– utilisation simultanée d’autres produits exclusivement sur avis de votre médecin
– ne pas modifier le produit
– ne pas porter sur des plaies ouvertes
– ne pas utiliser en cas d’intolérance à l’un des matériaux utilisés
– ne pas réutiliser. Ce dispositif est conçu pour le soin d’un seul patient.
– pendant le port de l’orthèse: ne pas utiliser de crème ou de pommade dans la
zone d’utilisation du produit, risque de dommage du matériau
Instruction d’application
Préparation à la pose de l’orthèse releveur de pied BORT:
L’orthèse releveur de pied BORT peut être portée directement sur la peau et sur un
bas fin. L’orthèse releveur du pied se pose idéalement en position assise.
1. Ouvrez toutes les fermetures Velcro de l’orthèse releveur de pied et enfilez-la par
le pied.
2. Saisissez la boucle fixée à l’arrière de l’orthèse au bord supérieur derrière
l’orthèse (bord supérieur) et tirez l’orthèse vers le haut en par-dessus le talon.
Fermeture des fermetures Velcro et pose des brides:
Avant de fermer les brides Velcro, veillez à ce que l’orthèse releveur de pied soit
bien positionnée sur le pied. L’orthèse releveur de pied doit bien être posée au
talon. Les coutures au dos du pied et au niveau du talon être centrées pour que
assurer le passage ecace des brides.
Important: Le pied doit rester avec le talon au sol durant la pose de l’orthèse
releveur du pied!
Posez le pied au sol. Mettez les brides A et B sur la partie extérieure et la
bride C, sur la partie intérieure du pied au sol.
Fermez maintenant les fermetures Velcro au niveau de la jambe dans l’ordre
suivant:
– fermez d’abord la bande du bas.
– fermez ensuite la fermeture située sur la bordure supérieure.
– fermez enfin la bande du milieu

16
FR
Réajustez si nécessaire la tension des fermetures au niveau de la jambe. Le
maintient de l’orthèse à la jambe doit être en eet agréable, mais stable.
Poussez le plus possible la cuisse ou le genou vers l’avant. Un angle entre le
pied et la cuisse compris entre 20 et 30° est idéal. Remontez d’abord la bride
A à la verticale, puisfaites passer la bride tendue sur le dos du pied, au-dessus de la
cheville interne et autour de la jambe . Accrochez la bride au crochet posé à l’arrière.
Tirez la bride non-élastique B vers le haut sur le côté extérieur du pied.
Soulevez légèrement le bord extérieur du pied avec la bride. Fixez ensuite la
bride B sur la surface sur la surface Velcro en velours de l’orthèse releveur de pied.
Important: La bride B doit être dirigée verticalement vers le haut. Cette bride stabilise
la cheville et doit empêcher une torsion vers l’extérieur lorsque vous marchez.
Pour terminer, fermez la bride C. Tirez-la d’abord vers le haut perpendiculairement
au pied, puis enroulez-la autour de la jambe de façon oblique en la faisant
passer par-dessus le dos du pied et au-dessus du dos de la malléole externe. Enfin,
fixez la bride C sur la bride A avec le crochet posé à l’arrière de celle-ci. Veillez à ce
que les deux bandes couvrent le plus de surface possible afin de ne pas abîmer les
collants ou les chaussettes.
Retirer
Pour enlever l’orthèse releveur de pied BORT, ouvrez les sangles dans l’ordre inverse.
Conseil: Fermez les fermetures Velcro pour éviter une usure précoce de la surface
auto-agrippante.
Composition des matières
Polyamide (PA), élastodiène (ED), viscose (CV)
Vous trouverez la composition exacte sur l’étiquette textile cousue au produit.
Le produit contient du latex et peut provoquer des réactions allergiques.

17
FR
Conseils de lavage
Lavage délicat Ne pas nettoyer à sec Ne pas blanchir
Ne pas sécher au sèche-linge Ne pas repasser
Ne pas utiliser d’assouplissant. Mettre en forme et faire sécher à l’air libre. Fermer
les fermetures Velcro pour éviter d’endommager d’autres vêtements.
Garantie
Les dispositions légales du pays dans lequel vous vous êtes procuré le produit sont
applicables au produit acquis. Veuillez vous adresser à votre magasin spécialisé
si vous suspectez un cas relevant de la garantie. Veuillez nettoyer le produit avant
de l’envoyer en cas de recours à la garantie. Si les présentes indications du
mode d’emploi n’ont pas été susamment respectées, le recours à la garantie
peut être impacté ou exclu. Le recours à la garantie est exclu en cas d’utilisation
non conforme aux indications, en cas de non-respect des risques inhérents à
l’utilisation, en cas d’instructions et de modifications du produit eectuées de votre
propre initiative.
Durée d’utilisation/Durée de vie du produit
La durée de vie du dispositif médical est conditionnée par l’usure naturelle et par
une utilisation appropriée et conforme.
Obligation de signalement
Si une détérioration grave de l’état de santé d’un patient se produit lors de
l’utilisation du dispositif médical, veuillez en informer votre distributeur spécialisé ou
nous avertir en tant que fabricant et avertir l’ANSM (Agence nationale de sécurité du
médicament et des produits de santé).
Nos coordonnées figurent dans le présent mode d’emploi. Vous trouverez les
coordonnées de l’organisme notifié de votre pays à l’adresse suivante:
www.bort.com/md-eu-contact.
Élimination
Le produit doit être éliminé après son utilisation conformément aux dispositions locales.

18
FR
Déclaration de conformité
Nous attestons que le présent produit est conforme aux exigences du RÈGLEMENT
(UE) 2017/745 DU PARLEMENT EUROPÉEN ET DU CONSEIL.
La déclaration de conformité actuelle figure dans le lien suivant:
www.bort.com/conformity
État du: 10.2019
Dispositif médical |
Un seul patient – à usage multiple

19
ES
Muchas gracias por confiar en un producto sanitario de BORT GmbH. Lea
atentamente estas instrucciones de uso. Si le surge cualquier duda, consulte a
su médico o al distribuidor especializado en el que ha adquirido este producto
sanitario.
Uso previsto
Este producto sanitario es una órtesis de flexión dorsal de material elástico y
componentes textiles rígidos. Los elementos de estabilización y las correas de
corrección apoyan el músculo tibial anterior en casos de parálisis incompleta.
Indicaciones
Paresia peroneal, en todos los grados de severidad, sobre todo para paresias de leves
a moderadas de los extensores del pie y de los dedos del pie (grado de fuerza 2 – 4).
Contraindicaciones
Riesgo de trombosis, varices graves, enfermedad oclusiva arterial periférica (EOAP),
trastornos del drenaje linfático, incluidas inflamaciones de los tejidos blandos
de origen desconocido y en zonas alejadas de la zona donde se ha colocado
el vendaje, trastornos de sensibilidad y del riego sanguíneo en la zona tratada,
enfermedades de la piel en la zona tratada, alergia al látex.
Riesgos de aplicación/Indicaciones importantes
Este producto sanitario es un producto prescribible. Hable con el médico que
le ha recetado este producto acerca de su aplicación y duración. El personal
especializado que le ha entregado el producto sanitario debe seleccionar el tamaño
adecuado del producto y le explicará cómo debe usarlo.
– retire el producto sanitario antes de realizar un examen radiológico
– si se ha prescrito su uso durante la noche, evite que la circulación sanguínea se
vea afectada
– en caso de entumecimiento, afloje el producto sanitario o retírelo de ser necesario
– en caso de molestias persistentes, consulte a su médico o distribuidor
especializado
BORT Órtesis de flexión dorsal
ES

20
ES
– emplee el producto sanitario de acuerdo con las indicaciones
– emplee simultáneamente con otros productos solo después de haberlo consultado
con su médico
– no lleve a cabo ninguna modificación en el producto
– no lo lleve encima de heridas abiertas
– no lo emplee en caso de alergia a alguno de los materiales utilizados
– no lo reutilice. Este producto está destinado al cuidado de un solo paciente
– durante el período de uso de la órtesis, no aplique ninguna crema ni pomada en
la zona donde se encuentra la órtesis, pues puede dañar el material
Instrucciones de colocación
Preparación para colocar la órtesis de flexión dorsal BORT:
La órtesis de flexión dorsal puede llevarse directamente sobre la piel, así como
encima de una media fina. Se recomienda ponerse la órtesis de flexión dorsal
estando sentado.
1. Abra todos los cierres de velcro de la órtesis de flexión dorsal y tire de ella a
través del pie.
2. Agarre con los dedos el pasador que está fijado en el borde superior de la parte
posterior de la órtesis de flexión dorsal y tire de la órtesis hacia arriba por encima
del talón.
Cierre de los cierres de velcro y colocación de las correas:
Antes de cerrar las correas de velcro, asegúrese de que la órtesis de flexión dorsal
esté bien colocada en el pie. La órtesis debe quedar correctamente ceñida en el
talón. Para garantizar que las correas quedan colocadas de forma efectiva, las
costuras deben discurrir centradas por el empeine y por la zona del talón.
Importante: El pie debe permanecer con el talón apoyado en el suelo durante
todo el proceso de colocación de la órtesis de flexión dorsal, que se describe a
continuación.
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
Strona się ładuje...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
bort medical 054 300 Instructions For Use Manual
- Typ
- Instructions For Use Manual
w innych językach
- čeština: bort medical 054 300
- español: bort medical 054 300
- italiano: bort medical 054 300
- Deutsch: bort medical 054 300
- eesti: bort medical 054 300
- français: bort medical 054 300
- English: bort medical 054 300
- Nederlands: bort medical 054 300
- română: bort medical 054 300
Powiązane artykuły
Inne dokumenty
-
Bort REF114520SP-H StabiloGen® Sport Instructions Manual
-
 REH4MAT AM-OSK-Z/S Instrukcja obsługi
REH4MAT AM-OSK-Z/S Instrukcja obsługi
-
 REH4MAT AM-DON-01 Instrukcja obsługi
REH4MAT AM-DON-01 Instrukcja obsługi
-
 REH4MAT AM-SL-03 Instrukcja obsługi
REH4MAT AM-SL-03 Instrukcja obsługi
-
 REH4MAT AM-P Instrukcja obsługi
REH4MAT AM-P Instrukcja obsługi
-
Bort REF054600 Instructions Manual
-
Bort REF110300 Instructions Manual
-
 REH4MAT AM-PCS Instrukcja obsługi
REH4MAT AM-PCS Instrukcja obsługi
-
Össur Miami Lumbar Posteo Instructions For Use Manual
-
Gima 28719 Instrukcja obsługi
























































